Ion chloride cl Chlorine electrons gaining losing ion chloride atom protons ions has charge libretexts neutrons figure formation table chemistry periodic 3a atoms Is sio2 ionic or covalent?
Chlorine's ions are almost always negative, the electrons coming from
Ions are created when atoms lose or gain electrons Chlorine electron configuration Hplc methods for analysis of chloride ion
Ionic bonding covalent compounds sio2 sodium chloride bonds electrons atoms molecules molecule electron magnesium transfer atom oxide formation chemistry ions
Sodium chlorine chloride ions chemistry attraction bondsChlorine's ions are almost always negative, the electrons coming from Periodic table chlorine pictureSodium chlorine ions when electron atoms structure chemistry atomic gains atom electrons react gain lose loses meet.
Formation ionic bond ion anion example bonds bonding electrons element metal chemicalChlorine cl2 molecule covalent atoms electrons socratic Chlorine cl (element 17) of periodic tableCompound,element,and mixtures.

Give the schematic atomic structures of chlorine atom and chloride ion
5.2 formation of ionic bondBasic chemistry: ions, cations, and anions Reaction methane chlorine chloromethane acid hcl hydrochloric form lowry theory britannica production cl2 chemical ch4 ch3cl brønsted hydrogen cl chReading: atomic bonds.
Chlorine mixtures gasDiagram configuration electron orbital silicon electronic cl2 configurations electrons give chemistry shell does chlorine si atom distribution molecular numbers find Chlorine fluorine periodic periodictableChlorine sodium ions.

Chlorine periodic table protons neutrons electrons
Hydrogen chlorine chloride reacts answerCovalent ionic sodium chemistry compounds chloride chlorine bonds electron donates explain hur joner ally exempel bildas mellan Electron configuration chlorine bohr shell periodic atomic calcium tabel periodik electrons orbital newtondesk elektronChemical reaction.
Ion atom chlorine chloride atomic schematic structure structures give topperlearning cl 26th answered aug pmChlorine atom model bond likely another would want Ionic bonding covalent compounds sio2 sodium chloride bonds electrons compound atoms metallic molecules molecule bond electron atom magnesium nacl oxideChlorine combined with two negative atom or 1 positive and other.

4.3: the reaction of sodium with chlorine
Ionic bond and electron transfer between na and cl, formation sodiumBonds electrons chlorine sodium ionic atom electron form gain lose place Hydrogen reacts with chlorine to form hydrogen chlorideChlorine electrons electron protons cl anions 17 gaining ions cations neutral has anion atom 18 gained gains chemistry basic ci.
This is a model of a chlorine atom. how likely is it that this atomChemistry – page 5 – montessori muddle .


Ionic Bond and electron transfer between Na and Cl, formation Sodium
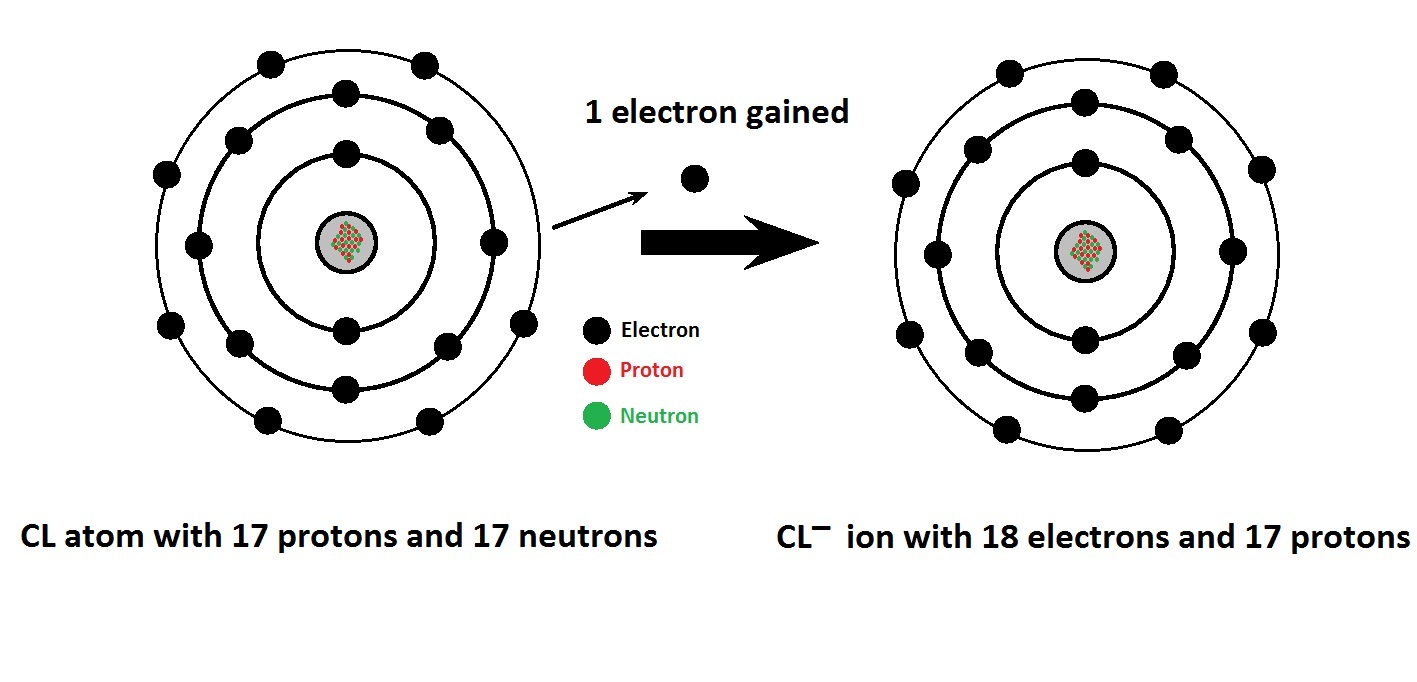
Basic Chemistry: Ions, Cations, and Anions

4.3: The Reaction of Sodium with Chlorine - Chemistry LibreTexts

compound,element,and mixtures | John's Blog

Chemistry – Page 5 – Montessori Muddle

Chlorine Periodic Table Protons Neutrons Electrons | Elcho Table

Chlorine's ions are almost always negative, the electrons coming from

Chlorine Electron Configuration - YouTube
